phosphorus valence electrons
Can phosphorus have 9 valence electrons. Since phosphorus is in group 15 it has 5 valence electrons.
 |
| Phosphorus Valence Electrons Phosphorus Valency P With Dot Diagram |
What is the atomic number of phosphorus.
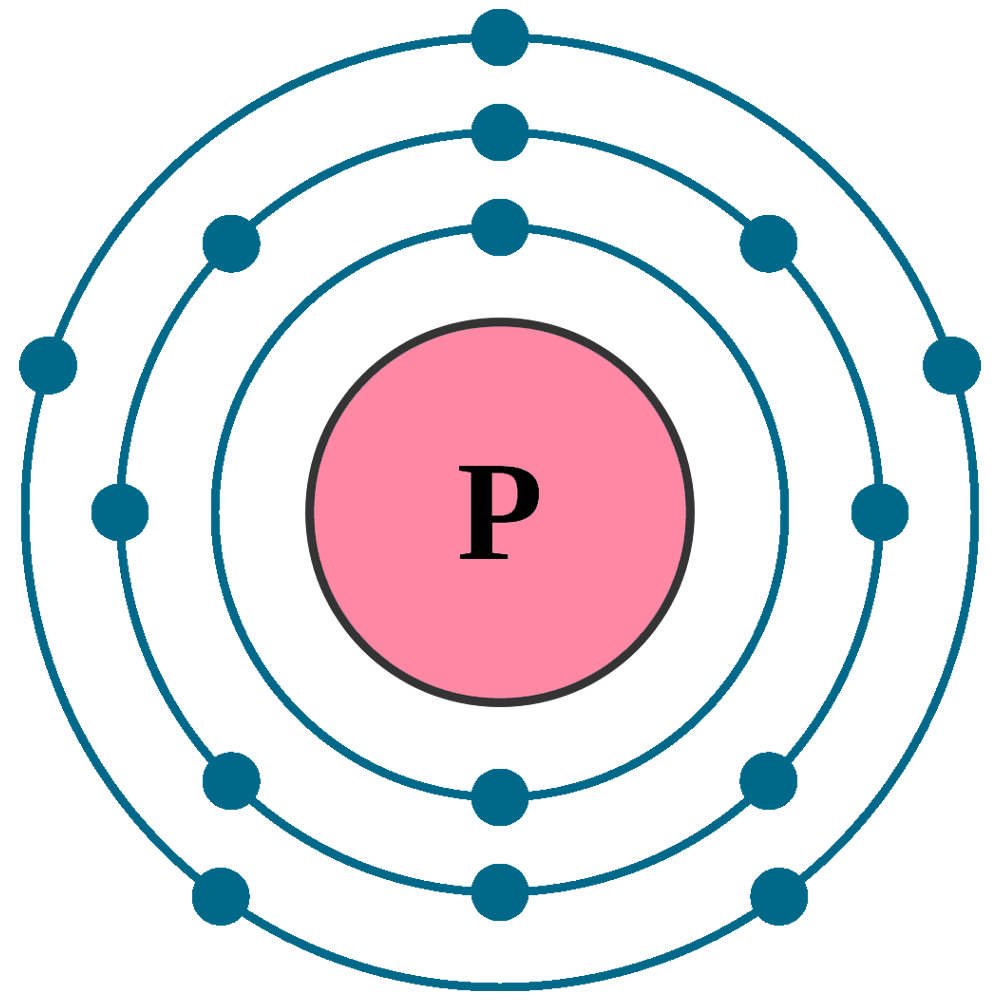
. These valence electrons participate in bond formation. Calcium is a metal and Phosphorus is a non metal so an ionic compound will be formed and the metal will give its electrons to non metal. And the total number of electrons present in this energy level. It is shown below.
The ground-state electron configuration of the Phosphorus P atom is 1s22s22p63s23p3. Unlike atoms from periods one and two that only have. This article explains in detail the valence electrons for. It is shown below.
In the periodic table phosphorus lies in group 15 and oxygen lies in group 16. Summary The electron configuration of Phosphorus in terms of the shell or orbit is 2 8 5. Hence phosphorus has five valence electrons and oxygen has six valence electrons. There are two ways to find the number of valence electrons in Phosphorous P.
Determine the valence electrons of phosphorus and hydrogen atoms The atomic number of phosphorus is 15. Phosphorus is the element in group 15. Here the given molecule is PCl5 Phosphorus pentachloride. For finding out the total number of valence electrons for POCl3 we find the valence electrons of all the atoms.
The last shell of phosphorus has three unpaired electrons so the valency of phosphorus is 3. It is shown below. Through its valence electrons phosphate forms bonds. Calculate the total number of valence electrons.
How Many Valence Electrons Does Phosphorus Have. Phosphorus is an element which is part of Group 15 formally known as the Pnictogen group and is directly below the nitrogen atom. You can see in the electron configuration of phosphorus 1s2 2s2 2p6 3s2 3p3 that the highest energy level is 3. Determine the total number of.
2 Using Electron Configuration First write electron configuration of phosphorus The electron configuration of. The first is to use the Periodic Table to figure out how many electrons Phosph. The elements that have 5 6 or 7 electrons in the last shell receive the electrons in the last shell during bond formation. One can also reckon it as the.
Its symbol is P. The last electron of phosphorus enters. Therefore the valence electrons of phosphorus are 5. Well in simple and straightforward words Phosphorus has five valence electrons.
Calcium and Phosphorus lewis dot structure. Determine the valence electrons of phosphorus and chlorine atoms The atomic number of phosphorus is 15. In order to draw the lewis structure of PCl5 first of all you. Therefore the valence electrons of phosphorus are five.
As was mentioned before a neutral Phosphorus Atom. Determine the total number of valence. Determine the valence electrons of phosphorus and chlorine atoms The atomic number of phosphorus is 15. Determine the total number of valence.
 |
| Solved What Is The Valence Electron Configuration For The Chegg Com |
 |
| Solved Questions Consider Phosphorus Which Has An Atomic Chegg Com |
| N Type Semiconductor Toshiba Electronic Devices Storage Corporation Asia English |
 |
| Makethebrainhappy How Many Valence Electrons Are In An Atom Of Phosphorus |
 |
| The Number Of Unpaired Valence Electrons In An Atom Of Phosphorus I Youtube |
Posting Komentar untuk "phosphorus valence electrons"